Tuberculosis testing
Methods for testing latent TB
Two types of latent TB tests utilize the immune response to detect TB: skin tests (tuberculin or specific TB antigens) and interferon-gamma release assays (IGRAs). The skin tests have significant drawbacks, including the requirement for two clinic visits, specialized staff training, potential reactivity to Bacillus Calmette-Guérin (BCG) vaccination leading to false positives, and only moderate sensitivity and specificity, potentially resulting in missed latent TB cases.4
In contrast, IGRAs function by identifying TB-specific effector T cells from the blood in vitro for latent TB detection. These tests are conducted in the laboratory, necessitate only one patient visit, and crucially remain unaffected by BCG vaccination.5, 6
There are two different IGRAs:
- ELISPOT IGRA (the T-SPOT.TB test) - Where peripheral blood mononuclear cells (PBMCs) are isolated, washed and counted to purify them from the whole blood prior to cell stimulation.
- ELISA/CLIA IGRAs - Where the cell stimulation is performed in the whole blood.
Why choose the T-SPOT.TB test?
- High sensitivity and specificity providing accurate results5
- Maintains performance in the immunosuppressed 9,13
- Low indeterminate results, few repeat tests 12
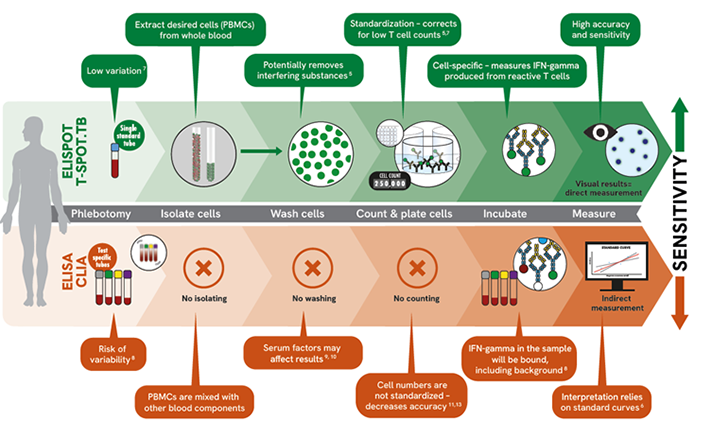
The T-SPOT.TB test has three crucial steps that have recently been acknowledged by the WHO for ensuring reproducibility and mitigating the impact of pre-analytical variables.7 These steps include isolating, washing, and counting the PBMCs before the test is performed. Shifting from traditional whole blood sample testing, the T-SPOT.TB test provides precision and reliability, allowing more control in your TB infection testing.
Here's how:
- Only one standard blood collection tube is needed throughout the T-SPOT.TB process
- Isolates PBMCs from whole blood, washes and counts them:
- Isolate cells: Extract the desired cell population (PBMCs) from whole blood
- Wash cells: Enables removal of potential interfering substances from whole blood
- Count cells: Ensures the required number of cells are used to produce reportable and accurate results regardless of individual patient cell counts
- Directly visualize the results without relying on interpretations from standard curves
Key differences with IGRAs
Explore our solutions
Products may not be licensed in accordance with the laws in all countries. Please check with your local representative for availability. Revvity, Inc. does not endorse or make recommendations with respect to research, medication, or treatments. All information presented is for informational purposes only and is not intended as medical advice.
References
- Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO.
- World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management.
- World Health Organization. The End TB Strategy. Geneva; 2014. who.int/teams/global-tuberculosisprogramme/theend-tb-strategy Accessed: 2-AUG-23
- Schluger NW, Burzynski J. Recent advances in testing for latentTB.Chest. 2010 Dec; 138(6): 1456–63.
- Oxford Immunotec T-SPOT.TB Package Insert PI-TB-IVD-UK-v5. Abingdon, UK. November 2023.
- QuantiFERON®-TB Gold Plus ELISA Kit Instructions for Use. L1123669_R3_IVDr_QF_ELISA_ROW_0323_FINAL. March 2023.
- World Health Organization. WHO operational handbook on tuberculosis. October 1, 2022.
- Banaei N, Gaur RL, Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? Journal of clinical microbiology. 2016; 54(4): 845–850.
- Wong SH, Gao Q, Tsoi KK, Wu WK, Tam LS, Lee N, Chan FK, Wu JC, Sung JJ, Ng SC. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016 Jan.
- Bèlard, et al. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflammatory Bowel Diseases, Volume 17, Issue 11, 1 November 2011, Pages 2340–2349.
- Komiya K, Ariga H, Nagai H, et al. Impact of Peripheral Lymphocyte Count on the Sensitivity of 2 IFN-γ Release Assays, QFT-G and ELISPOT, in Patients with Pulmonary Tuberculosis. Intern Med. 2010; 49(17): 1849–55.
- Rego K, et al. Utility of the T-SPOT®.TB test’s borderline category to increase test resolution for results around the cut-off point. Tuberculosis. 2018; 108:178 185.doi:10.1016/j.tube.2017.12.005.
- Clark SA, Martin SL, Pozniak A, et al. Tuberculosis antigenspecific immune responses can be detected using enzymelinked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007; 150(2):238–244.
Questions?
We’re here to help.
Contact us Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.