T-SPOT technology
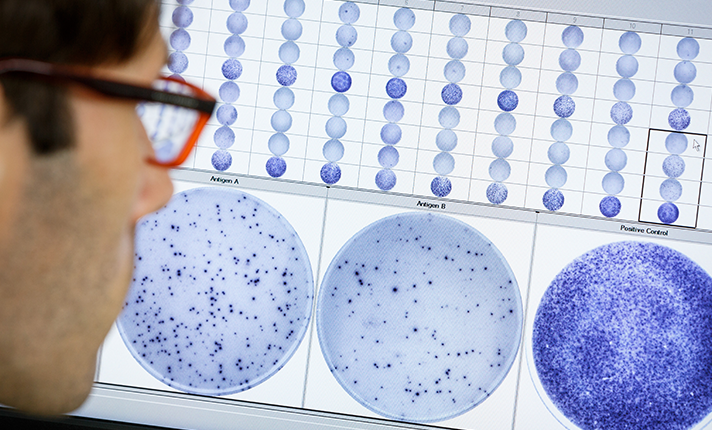
We have over 20 years of experience in T cell immunology and accurately measuring T cell responses using our T-SPOT technology. By leveraging the ELISPOT methodology to perform an interferon-gamma release assay (IGRA), it offers a refined analysis of individual immune responses to specific antigens.
The technology includes 3 crucial steps:
- Cell isolation: Extract the desired cell population (PBMCs) from whole blood
- Cell washing: Enables removal of potential interfering substances from whole blood
- Cell counting: Ensures the required number of cells are used to produce reportable and accurate results regardless of individual patient cell counts
Through this innovative technology, we demonstrate our commitment to pushing the boundaries of healthcare and harnessing the power of the immune system to enhance diagnostic accuracy and improve patient outcomes.

Tuberculosis
For every person with active tuberculosis (TB), many more have a latent TB infection. Find out how Revvity are advancing TB management with the T-SPOT.TB test.
For every person with active tuberculosis (TB), many more have a latent TB infection. Find out how Revvity are advancing TB management with the T-SPOT.TB test.

Cytomegalovirus
Cytomegalovirus (CMV) is an opportunistic virus and common cause of morbidity and mortality in solid organ and hematopoietic stem cell transplant patients. To help assess a patient’s level of anti-CMV cell mediated immunity, Revvity have developed the T-SPOT.CMV test.
Cytomegalovirus (CMV) is an opportunistic virus and common cause of morbidity and mortality in solid organ and hematopoietic stem cell transplant patients. To help assess a patient’s level of anti-CMV cell mediated immunity, Revvity have developed the T-SPOT.CMV test.

SARS-CoV-2
CE marked for IVD use, the T-SPOT.COVID test was developed for use as an aid in identifying and monitoring individuals with a T cell immune response to COVID-19 vaccination or SARS-CoV-2 infection.
CE marked for IVD use, the T-SPOT.COVID test was developed for use as an aid in identifying and monitoring individuals with a T cell immune response to COVID-19 vaccination or SARS-CoV-2 infection.
Products may not be licensed in accordance with the laws in all countries. Please check with your local representative for availability. Revvity, Inc. does not endorse or make recommendations with respect to research, medication, or treatments. All information presented is for informational purposes only and is not intended as medical advice.
References:
- National Institute of Allergy and Infectious Diseases. Overview of the Immune System.
- Abbas AK, et al. Cellular and Molecular Immunology. 9th edition. Elsevier; 2017.
Questions?
We’re here to help.
Contact us Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.





























