Accelerating therapeutic development with pharmaceutical companies in common and rare diseases
Our strategic partnerships with pharmaceutical companies are geared towards facilitating omics discoveries, fast-tracking the development of treatment for genetic diseases, and supporting patients who have rare diseases.
At Revvity, our laboratories are equipped with the latest instruments and platforms for multi-omics testing. Our focus on cutting-edge technology, combined with our extensive expertise, global presence, and commitment to providing exceptional customer experience makes Revvity Omics a partner of choice for any project.
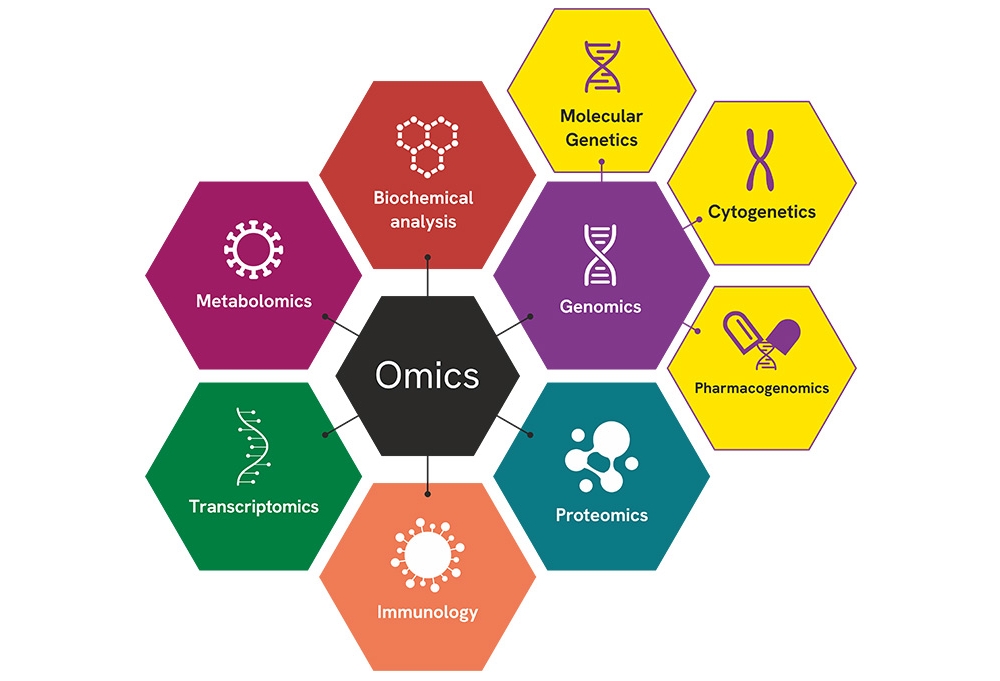
Inherent expertise with cutting-edge technologies across multiple fields
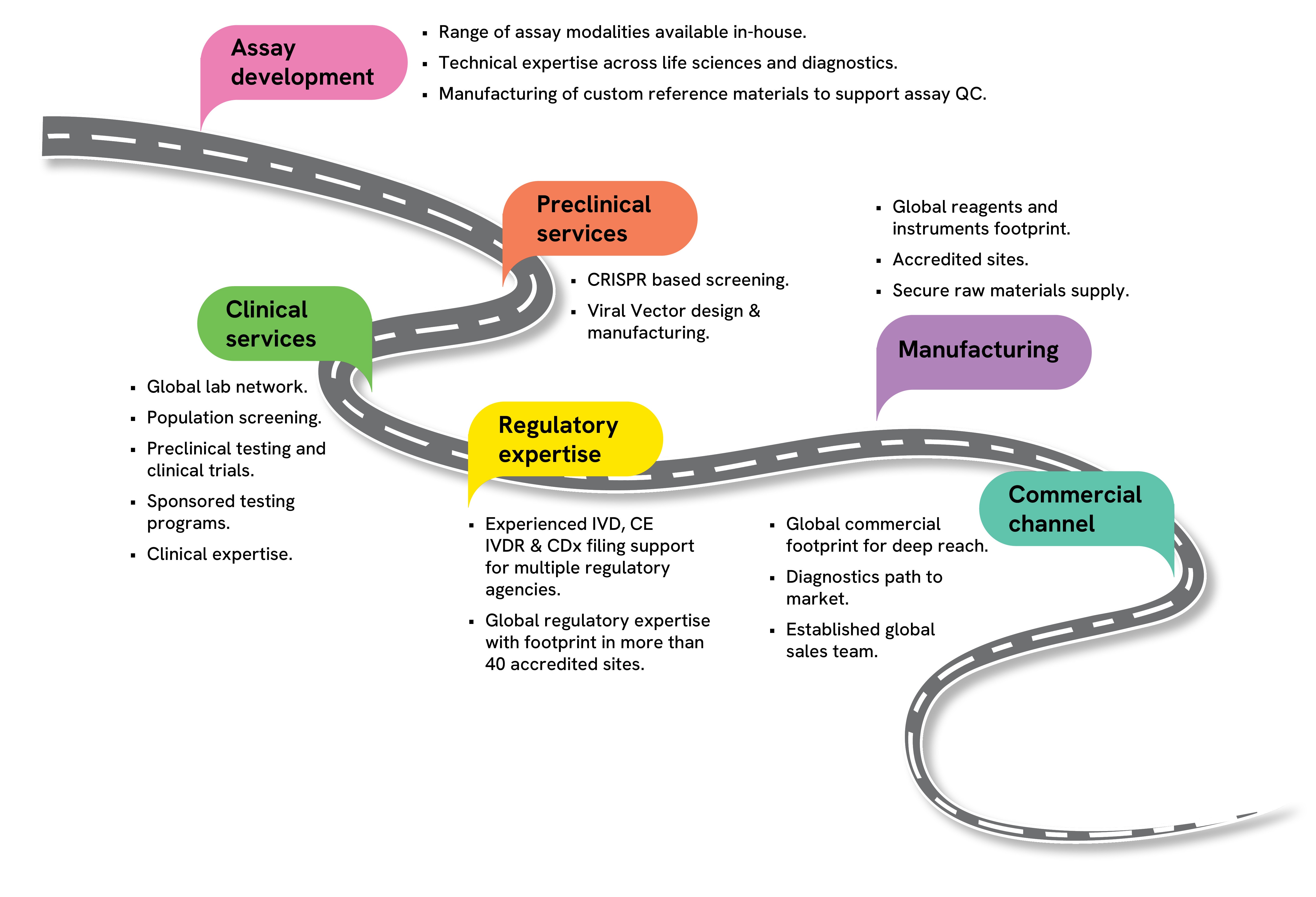
Supporting clients through their journey of research and therapeutic development
Bespoke scientific consultancy with multi-omics experts
Custom Research Use Only (RUO) assay development and optimization with experts in:
- Genomics
- Biochemical genetics
- Cytogenetics
- Transcriptomics
- Proteomics
- Immunology
Custom Research Use Only (RUO) assay development and optimization with experts in:
- Genomics
- Biochemical genetics
- Cytogenetics
- Transcriptomics
- Proteomics
- Immunology
Clinical services through accredited global laboratory network:
- Sponsored testing programs for patient identification
- Testing to support clinical trials
- Design, manufacturing, and distribution of sample collection kits
- Sponsored testing programs for patient identification
- Testing to support clinical trials
- Design, manufacturing, and distribution of sample collection kits
Ensuring success of programs through dedicated program management
Other key services included:
- Regulatory and compliance support
- Research Use Only (RUO) kit manufacturing and distribution
Other key services included:
- Regulatory and compliance support
- Research Use Only (RUO) kit manufacturing and distribution
Current programs include
Products mentioned are developed by, and performance characteristics are determined by Revvity Omics, a wholly owned subsidiary of Revvity, Inc. Revvity Omics laboratory is CAP-accredited and certified under the Clinical Laboratory Improvement Amendments (CLIA) as qualified to perform high complexity clinical laboratory testing. Products mentioned have not been cleared or approved by the U.S. Food and Drug Administration; safety and effectiveness of the products has not been established.
Questions?
We’re here to help.
Contact us Please note that product labeling (such as kit insert, product label, and kit box) may be different compared to the company branding. Please contact your local representative for further details.